



Helps ensure that your patient receives the intended dose.*
*Use an accurate dose-measuring cup to get the right dose of CUVPOSA.1

No mixing or pill crushing.† No more asking your patient to finish their food so they receive the proper dose.
†Give CUVPOSA at least 1 hour before or 2 hours after meals.1

Easy to titrate based on your patient's needs to deliver an individualized dose.
CUVPOSA was evaluated in a multi-center, randomized, double-blind, placebo-controlled, parallel, eight-week study for the control of pathologic drooling in children (Study 1). The study enrolled 38 subjects aged 3-23 years; thirty-six subjects were aged 3-16 years and two patients were greater than 16 years.
The subjects were male or female, weighed at least 13 kg (27 lbs), and had cerebral palsy, mental retardation, or another neurologic condition associated with problem drooling defined as drooling in the absence of treatment so that clothing became damp on most days (approximately five to seven days per week).
Subjects were randomized in a 1:1 fashion to receive CUVPOSA or placebo. Doses of study medication were titrated over a 4-week period to optimal response beginning at 0.02 mg/kg three times a day increasing doses in increments of approximately 0.02 mg/kg three times per day every 5-7 days, not to exceed the lesser of approximately 0.1 mg/kg three times per day or 3 mg three times per day.
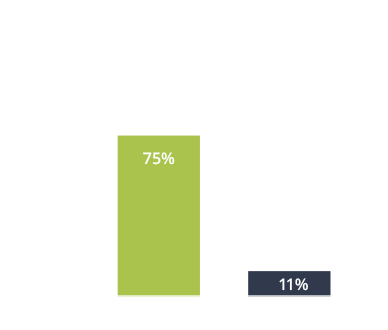
CUVPOSA® was tested in a clinical trial. At the end of this study—8 weeks after starting treatment—75% of patients who were given CUVPOSA saw a reduction in drooling. Only 11% of patients who received the placebo‡ saw a reduction after 8 weeks. And patients treated with CUVPOSA also saw some reductions as early as the fourth week of treatment.1
‡In the clinical trial, half the patients, chosen at random, received CUVPOSA; the other half received a placebo (similar-looking and -tasting liquid with no medicine in it). Neither the patients nor the doctors knew who got which until the trial was completed.
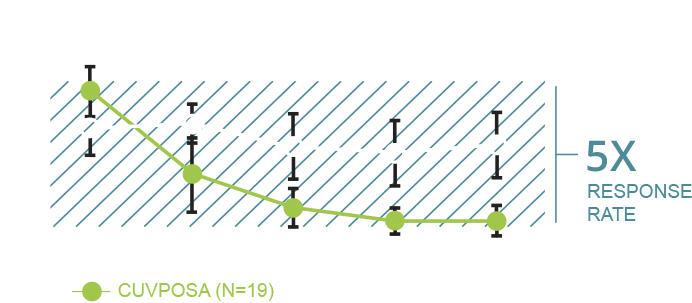
To measure the amount of drooling before and after treatment, the doctors used the modified Teacher’s Drooling Scale (mTDS). It provides a numerical score based on how often a child drools and how severe it is. The scale goes from 1 to 9. One is the lowest level of drooling; 9 is the highest.1
In this clinical trial, an improvement was defined as at least a 3-point reduction in the drooling scale (eg, the patient started at 7 and ended at 4).
Mean (±2 standard errors) mTDS Scores1
Nine-Point mTDS1
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a longer, 24 week, open-label study of 137 subjects, the most commonly reported adverse reactions were similar to those seen in the placebo-controlled clinical trial.1
CUVPOSA (glycopyrrolate) oral solution is indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling (e.g., cerebral palsy).
CONTRAINDICATIONS
CUVPOSA is contraindicated in:
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
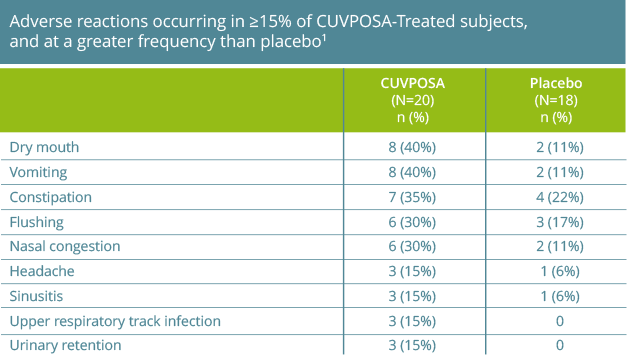
The most common adverse reactions reported with CUVPOSA are dry mouth, vomiting, constipation, flushing, and nasal congestion.
The most commonly observed adverse reactions reported by ≥15% of CUVPOSA-treated patients for the placebo-controlled clinical trial were:
The most commonly observed adverse reactions which occurred at a rate of <2% of CUVPOSA-treated patients in the open label study were:
Additional adverse reactions identified during post approval use of glycopyrrolate tablets include: loss of taste and suppression of lactation.
DRUG INTERACTIONS
Drugs Affected by Reduced GI Transit Time
Glycopyrrolate reduces GI transit time, which may result in altered release of certain drugs when formulated in delayed- or controlled-release dosage forms.
Amantadine
The anticholinergic effects of glycopyrrolate may be increased with concomitant administration of amantadine. Consider decreasing the dose of glycopyrrolate during coadministration of amantadine.
Drugs Whose Plasma Levels May be Increased by Glycopyrrolate
Coadministration of glycopyrrolate may result in increased levels of certain drugs.
Drugs Whose Plasma Levels May be Decreased by Glycopyrrolate
Coadministration of glycopyrrolate may result in decreased levels of certain drugs.
USE IN PREGNANCY
There are no available data in pregnant women for CUVPOSA to inform decisions concerning any drug-associated risks.
PEDIATRIC USE
CUVPOSA was evaluated for chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling. CUVPOSA has not been studied in subjects under the age of 3 years.